Bauxite
-
Bauxite Basics
History
Bauxite is the primary mineral for alumina and thus ultimately aluminium production. It is named after the town of Les Beaux in southern France, where the chemist Berthier first recognised it in 1821.Occurrence
Bauxite is widely distributed around the world, particularly in tropical areas. It is formed by weathering of aluminium rich rocks and the major deposits are therefore generally close to the surface.Reserve
World reserves of bauxite exceed 20 billion tonnes, with Guinea, Australia, Brazil, Vietnam and Jamaica holding over 50% of this figure.Production and Trade
In 2019 it is estimated over 400 million tonnes of bauxite was produced with 140 million tonnes being traded around the globe. China is the largest importer at ~100 million tonnes.Outlook
Increased demand for bauxite is set to continue as aluminium use in developing countries is well below that typical of more developed economies.Physical Form
Bauxite takes many physical forms including small red peas, red-yellow “soils”, and large, pale, hard stones.How to define an orebody
The main criterion for identification of an ore body as bauxite is that it should contain aluminium in the form of aluminium hydroxides, the minimum content being about 30% (measured as Al2O3).Bauxite – alumina
Typically between two and three tonnes of bauxite must be refined to produce one tonne of alumina.Waste from the refining process, so called “red mud”, is stored in large dams designed to prevent leakage of, and environmental damage from, the still highly caustic contents.
Typical Ore Pictures from Major Countries

Weipa bauxite (Source: http://aluminium.org.au/australian-industry/industry-description/australian-bauxite/weipa/)

Chinese bauxite (Source: CMGroup)

Indonesia Ore (Source: CMGroup)
Text Source: World Bauxite Resources, USGS ; CMGroup
-
Index Basics
The schematic shows the methodology of how the bauxite price is calculated based on actual shipments of bauxite imports into China.
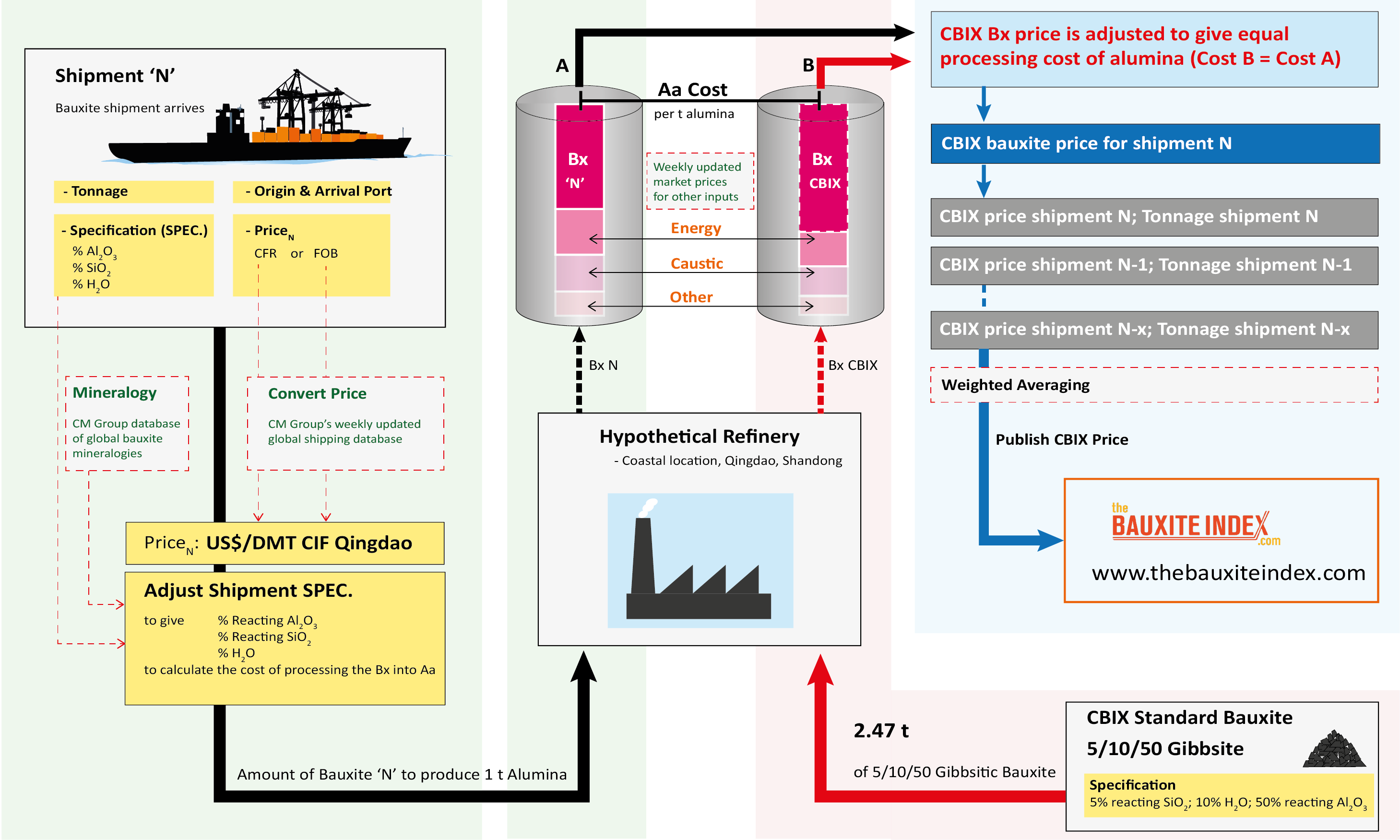
-
Mineralogy
The main alumina containing minerals that occur in bauxites are:
- Gibbsite
- Boehmite and
- Diaspore
Other gangue or impurity minerals typically found in bauxites include:
- Clays, typically kaolinite
- Quartz
- Iron oxide andiron hydroxy-oxide (hematite and goethite)
- Titanium dioxide in the form of anatase or rutile.
The mineralogy is very important as it dictates the refining conditions that must be used and has a large bearing on the economics of processing a particular bauxite.
Source: World Bauxite Resources, USGS, CMGroup
-
Alumina Minerals
Gibbsite:
- Chemical Name & Formula: Aluminium Hydroxide (or Tri-hydroxide), Al(OH)3
- Technical or Common Name(s): Alumina Trihydrate (or simply ‘Trihydrate’ or ‘Hydrate’), usually written in oxide notation as Al2O3.3H2O
- The most abundant economic source of alumina. It is also the intermediate product in the refining process.
- Requires the lowest refining temperature, typically 150oC
Boehmite
- Chemical Name & Formula: Aluminium Hydroxy-oxide, AlOOH
- Technical or Common Name(s): Alumina Monohydrate, usually written in oxide notation as Al2O3.H2O
- Requires higher refining temperatures, typically 240-260oC
Diaspore
- Chemical Formula: AlOOH, but usually written in oxide notation as Al2O3.H2O
- Has the same chemical composition as boehmite but a different crystal form. It does not occur in tropical bauxites but is of importance in Europe & China.
- Requires the highest refining temperatures to process, typically +260oC
Source: World Bauxite Resources, USGS, CMGroup
-
Impurities
There are many commercially important impurities in bauxites, however of these silica (which may be present in various forms) is the most important.
The rule of thumb is that, on a weight basis, any mass of silica that reacts in the refining process will cause an equal loss of caustic soda, and an equal loss of alumina to the red mud in the De-Silication Product (DSP). Thus, silica is a key determinant of any bauxite’s economic value.
Important impurities and their effects listed opposite. Some impurities, for example gallium, build up in the refinery liquor and are potentially an economic by-product. However due to a very small market for such metals very few refineries extract and sell them.
Kaolinite
(Kaolin, China Clay; Al2Si2O5(OH)4 or Al2O3.2SiO2.2H2O):
- A key source of reacting silica; reactive under all refining conditions.
Quartz (Silicon Dioxide; SiO2):
- An additional source of reacting silica in high temperature refineries.
Hematite (Iron Oxide; Fe2O3):
- Essentially passes through the refining process unchanged.
- Chemical Formula: Fe2O3
Goethite (Iron Hydroxy-oxide; FeOOH or Fe2O3.H2O):
- Often fine grained: associated with slow or poor settling of red muds.
- Due to aluminium ion substitution and solid solution into the goethite lattice it can be a minor contributor of alumina; accessible in high temperature refining.
Titanium Dioxide (Anatase, Rutile; TiO2):
- Associated with tenacious scales inside refining equipment. These reduce refinery efficiency and increase maintenance costs.
Organic Carbon
- Entrained vegetative materials in the bauxite partially decompose to form humic acids in the refinery liquor. These interfere with alumina precipitation and final product mechanical properties.
- Costly and difficult to remove organic carbon causes problems in some important bauxites (e.g. those in the Darling Range, Western Australia).
Sulphur
- Causes iron contamination and poor mechanical properties in the product alumina, extra equipment corrosion, reduced liquor C/S.
- Costly and difficult to remove; originates from sulphur minerals such as pyrite and/or organic materials in the bauxite.
Source: World Bauxite Resources, USGS, CMGroup
-
Types of Bauxite
Bauxite deposits are commonly referred to by a number of different terminologies relating to either their mineralogy or geological formation.
Alternately they may be described by likening them to other well-known deposits elsewhere around the globe.
According the to their mineralogy:
- Trihydrate or gibbsitic bauxite: consisting chiefly of gibbsite
- Mixed bauxite: typically consisting of significant proportions of both gibbsite and boehmite.
- Monohydrate bauxite: consisting mainly of boehmite or diaspore and
According to their geological formation
- Lateritic: formed in situ from weathering of aluminous parent rocks in tropical and temperature regions. Consisting mostly of gibbsite or a mixed gibbsite and boehmitecontent.
- Karst: partially transformed or transformed bauxite materials washed and accumulated in eroded limestone cavities where further transformation can occur. Commercially significant karst bauxites occur in Europe, the Middle East, China and Jamaica.
According to other well-known deposits:
- Suriname type: a pseudonym for trihydrateor gibbsitic bauxite
- European type: composed mainly of boehmite
- Jamaica type: applied to very fine grained high-iron gibbsitic bauxite containing minor quantities of boehmite.
Source: World Bauxite Resources, USGS
-
Bauxite Formation
Bauxite deposits are formed chiefly by weathering of aluminous rock; some have been transported to their present locations, but most are residual accumulations from which most other constituents of the parent rock, other than alumina, have been leached.
Bauxite deposits occur in rocks ranging in age from Precambrian to Holocene.
- Gibbsitic bauxite: Most deposits of this type are in the tropics and a few occur in the temperate belts, but the climate was probably tropical or subtropical at the time these formed. Nearly all are of Cenozoic age.
- Boehmitic bauxite: Deposits of this type occur chiefly in southern Europe, the USSRand Turkey. Most are associated with carbonate rocks of Jurassic and Cretaceous age, but a few are of Paleozoic age. Today most are north of the tropics, however they could have formed under tropical conditions.
- Mixed bauxites are associated with both the gibbsite and boehmite types. However, they tend to be more abundant in deposits of Paleozoic and Mesozoic ages than in younger rocks.
Formation Process
The processes involved in bauxite formation are more complex than shown below, but the following example for weathering of feldspar is indicative of the overall net formation sequence.
Step 1: Acidification of rainwater
CO2 + H2O → H2CO3
H2CO3 → HCO3– + H+
HCO3– → CO32- + H+
Step 2: Carbonic and humic acids (from soil) react with feldspar, leaching potassium and silica, and hydrating the alumino-silicate structure to form an illite clay:
3KAlSi3O8 + 2H+ + 12H2O → KAl3Si3O10(OH)2 + 2K+ + 6Si(OH)4
Step 3: Further leaching removes the remaining potassium, transforming illite to kaolinite:
2KAl3Si3O10(OH)2 + 2H+ + 3H2O → 3Al2Si2O5(OH)4 + 2K+
Step 4: Kaolinite is decomposed to form insoluble gibbsite and soluble hydrated silica:
Al2Si2O5(OH)4 + 5H2O → 2Al(OH)3 + 2Si(OH)4
Iron in the minerals is converted to the insoluble forms hematite and goethite. Titanium is transformed to anatase. Quartz and zircon are resistant to weathering.
The mixture of remnant minerals is called ‘laterite’ and is a common surface feature in tropical areas. If it is sufficiently high in alumina and low in silica, it is characterised as ‘bauxite’. Laterite bauxites account for most of the world’s major deposits of bauxite.
Weathering of limestone gives rise to eroded surface and sub-surface features (caves & depressions) which are together known as ‘karst’. If the voids are subsequently filled with minerals containing aluminium (eg. clays or laterite) then further weathering may occur leading to bauxitisation. The resulting deposits are known as karst bauxite. They are important in Europe, China and Jamaica.
Bauxite Chemical Composition
Although bauxite comprises a mixture of minerals, the composition is normally reported as the elemental analysis, expressed as metal oxides. This analysis is usually determined by X-Ray Fluorescence Spectrometry (XRF), though classical methods are also available.
The composition of bauxite samples from a range of deposits are tabulated below.
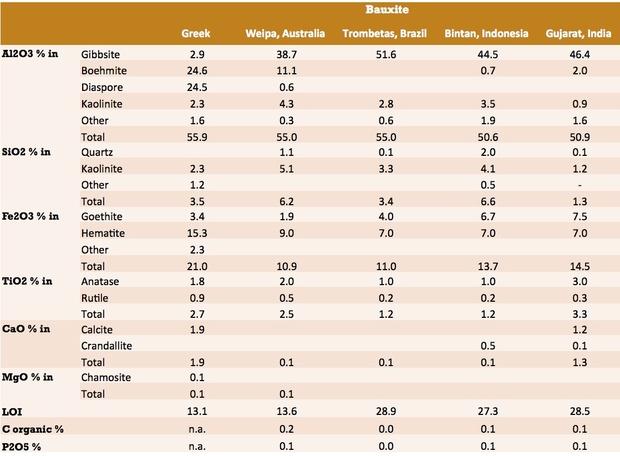
Source: USGS
-
Bauxite Occurrence
Based on a broad geological assessment the potential for bauxite mineralisationis quite common across the globe.Typically deposits in the tropics and southern temperate zones are lateritic deposits, while those in the northern temperate zones and beyond are monohydrate deposits, often associated with karst systems. World Bauxite – Key Countries Despite wide potential, most of the world’s known bauxite reserves and resources are concentrated in only 12 countries.
-
Bauxite Reserves
The top 5 countries, Guinea, Australia, Brazil, Vietnam and Jamaica, hold over 70% of the world’s documented bauxite reserves. Bauxite resources are estimated to be 55 billion to 75 billion tons, in Africa (32%), Oceania (23%), South America and the Caribbean (21%), Asia (18%), and elsewhere (6%).
Of these counties only Australia, Brazil and Jamaica have realised their potential as bauxite mining or alumina producing nations. While still a major bauxite producer sovereign risk has stunted development of the industry in Guinea to a fraction of its full potential.
Strict nationalistic policies in Vietnam have resulted in the industry only just beginning to emerge. Indonesia also possesses significant bauxite holdings and has become a major exporter to China.
World Bauxite Reserves – 2019
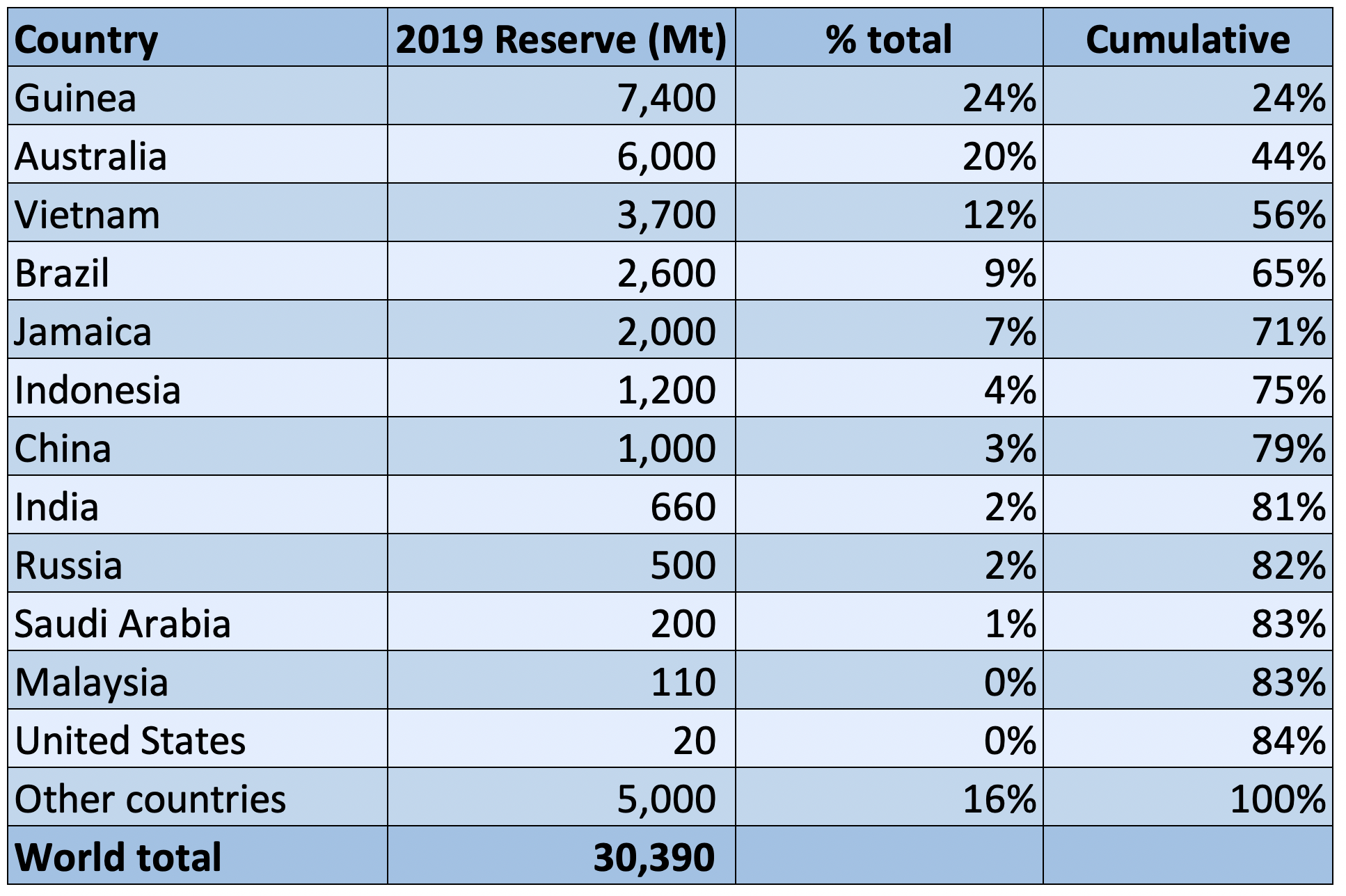
Source: USGS
World Bauxite Reserves – Key Counties
Brief details of the bauxite in key counties is provided below. More extensive analysis if these countries is provided in sections at the end of this document.
- Guinea: High alumina content. Major concentration areas: Lower Kindiaand Boke (around 5 billion), Central Labe (500 million), Gaoual (500 million); and Upper Dabola (around 1.9 billion).
- Australia: Abundant reserves of easily mined surface deposits. Major concentration areas include: 1) the Gulf of Carpentaria, 2) Darling Ranges, 3) Mitchell Plateau and Cape Bougainville.
- Brazil: 90% of Brazil’s bauxite is distributed in Para State in northern Brazil.
- Vietnam: Mainly distributed in DakLak, DakNong, Kon Tum and Lam Dong provinces in central and southern Vietnam. Deposits are mainly lateritic (Al2O3 36-39% or sedimentary (Al2O3 39-65%).
- India: Widely distributed, however 60% of all identified reserves are in Orissa and Andhra Pradesh along East Coast.
- Indonesia: Mainly distributed in Bangka Island, Belitung Island, West Kalimantan and Riau Province (Bintan Island).
Source: USGS; CMGroup
-
Bauxite Production
In 2019, around 370 million tonnes of bauxite were produced world-wide. The majority of this material was consumed in-country for the production of smelter grade alumna.
World Alumina Refinery and Bauxite Mine Production and Bauxite Reserves
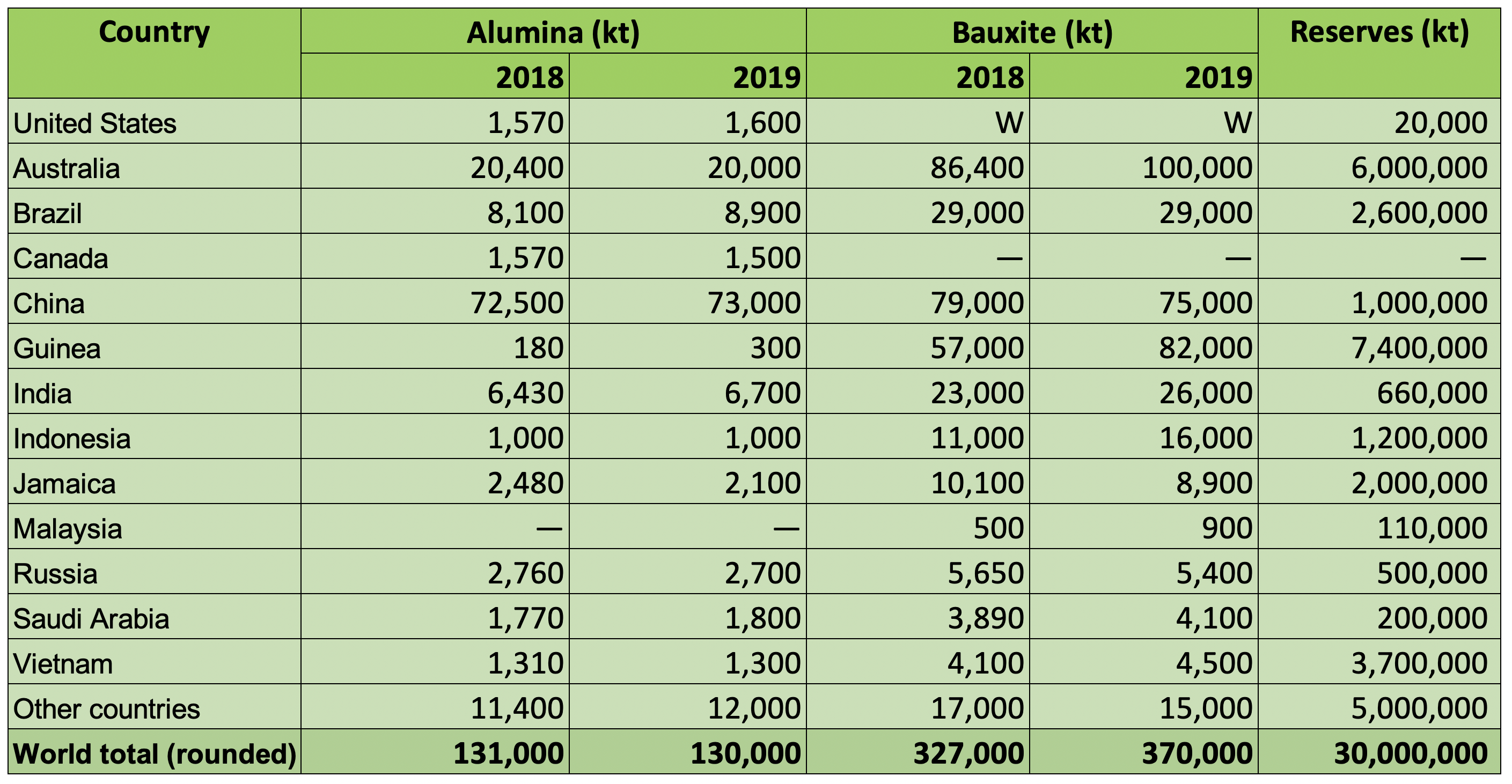
Source: USGS
-
Bauxite Trade
- World bauxite export trade is growing. In 2019, the total bauxite trade volume of the countries listed was over 120 million tonnes.
- Guinea has in recent years emerged as a major exporting country, with its 2019 exports to China (44.4 million tonnes), Ukraine, Ireland, Germany, India, etc.
- Australia has been a stable bauxite source to China, with over 36 million tonnes (dry) exported to China.
- Indonesia’s bauxite exports have been limited by its quota system since its relaxing of exports ban in 2017. Its annual exports to China in 2019 was 14.4 million tonnes (dry).
- China is the largest aluminium consuming and bauxite importing country in the world.
- In 2019, China’s imported over 100 million tonnes of bauxite, of which around 95% originates from Guinea, Australia and Indonesia.
-
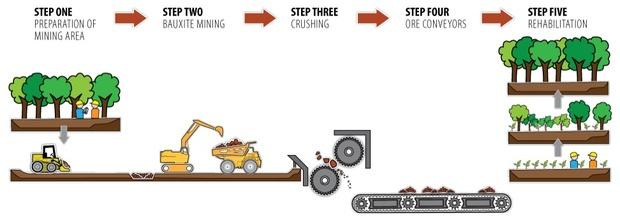
Bauxite Mining
Typical Bauxite Mining Process
- Vegetation is cleared and top soil is removed and set aside for rehabilitation
- Scrapers and excavators are used to remove the remaining overburden and expose the cap rock. Depending on the depth of the cap rock, it can be broken by blasting, or simply removed with scrapers and excavators
- The bauxite is then mined using excavators or loaders. Off road haul trucks transported the bauxite to the crusher. Several pits are usually mined simultaneously in order to supply the refinery with a consistent grade of ore.
- The mined out area is backfilled with the removed over burden, the set aside top soil is redeposited, then re vegetated with plants indigenous to the area.
Schematic of a typical mining process for lateritic bauxites
Bauxite comminution, beneficiation and transport
Comminution
- Crushing and grinding are used to break the ore down to a smaller size suitable for transport and or beneficiation.
Beneficiation
- Most bauxites considered economic require minimal or no beneficiation.
- Beneficiation of lateritic materials may involve dry screening to remove fine particles with higher silica contents, or simple washing to remove adherent clay minerals. More sophisticated beneficiation involving dense media separation and spirals has been practiced in Brazil.
- Froth flotation for silica removal is practiced in at least one refinery in China.
Transport
- Bauxite is transported by any number of means including off and on road trucks, rail, conveyor systems, ships and even as piped slurries.
Typical mining and transport equipment
The Haul trucks emptying run of mine (ROM) bauxite into crushers for the initial stages of comminution


Crushed bauxite transport via a covered conveyor

Source: Australian Aluminium Council; CMGroup; Picture Source: Alcoa.

BPI Calc - Bringing the Bauxite Index to your fingertips
BPI Calc is your essential bauxite‐price calculator, powered by CM Group’s latest Bauxite Price Index (BPI). Instantly estimate prices by grade and specification with real‑time and historical data, Value‑in‑Use (ViU) analysis and market insights.
Key Features
- Instant access to CM Group’s Bauxite Index Updates
- Spec‑driven price estimates
- Real‑time pricing with historical index tracking
- Advanced ViU estimation tools
- Alumina raw‑material and bulk‑freight insights
